

Head-bobbing of walking birds - a review
The original publication is available as PDF file download:
(Journal of Comparative Physiology A 193: 1177-1183 (2007))
Head-bobbing as observed, e.g. in pigeons and chickens means a rhythmic forward and
backward movement of the head during bipedal walking on the ground (Video 1). As
has been shown first by Dunlap and Mowrer (1930) the backward movement is based on
illusion: in this phase the head position is kept stable with regard to the environment
while the body moves continuously forward. In this way head movements during walking
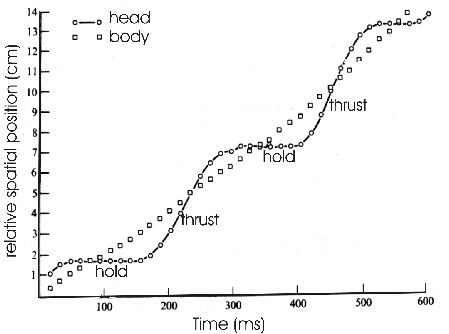
are characterized by a hold phase and a thrust phase (Fig. 1). The forward and backward
movements of the head are usually synchronized with the movements of the legs, i.e.
there is one head cycle per one step or two head bobs during a complete walking cycle.
Smaller birds often do not walk but hop. Since this includes a rapid head (and body)
movement and a stop of the head movement, it may be compared to the head-bobbing
of walking birds. Head-bobbing is of interest only in birds which regularly walk
on the ground. It cannot be expected in birds which primarily move in the air such
as many seabirds, swallows, humming birds and swifts.
Fig. 1: Movement of head and body of a pigeon shown as relative spatial position versus time
Video 1: Walking of a crane and a stork showing head-bobbing
Whiteside (1967) claims that the position of the eyes (frontal or lateral), and the
structure of the retina play an important role when it comes to the question whether
birds do bob their head or not. Birds with frontal eyes (raptors, caprimulgiforms
like nightjar; see Iwaniuk and Wylie 2006) do not show head-bobbing because they
dispose of a wide range of stereoscopic and thus depth vision (Whiteside 1967). However,
these birds usually catch their prey in the air and do not normally move on the ground.
Most birds have a central retinal area with an increased number of photoreceptors
(with or without a distinct fovea) and these birds bob their heads or hop (e.g. pigeons,
crows, blackbirds, and chaffinch). Birds with a horizontal retinal band of high photoreceptor
density, which are specialized to see all horizon to detect predators usually do
not bob their heads (e.g. gulls, flamingos, terns). However, black-headed gulls do
bob when feeding in the mud (Daanje 1951; Fujita 2006). If this band contains a distinct
fovea again bobbing occurs (e.g. turnstone, oystercatcher, coot). Pelicans have very
movable eyes and therefore do not bob (Whiteside 1967). Despite reports of the retinal
specializations of a variety of bird species (Rochon-Duvigneaud 1943; Walls 1963)
there is a need for detailed correlative studies to prove the hypotheses put forward
by Whiteside (1967). Furthermore, other mechanisms (kind of locomotion, feeding behavior)
may also be important in addition to eye position and retinal structure.
There is general agreement that foraging on the ground is indicative of head-bobbing
(Dagg 1977; Jimenéz Ortega 2005; Fujita and Kawakami 2003; Fujita 2004; 2006). However,
ducks and geese feed on land without head-bobbing. The stride length of steps seems
to be an important factor as has been pointed out already by Daanje (1951) and supported
experimentally by Fujita (2004; 2006). Herons do not bob when taking small steps
and the same is true for black-headed gulls (Daanje 1951; Fujita 2006) and for birds
which have short legs and take small steps (swallows, terns, ducks). These correlations
may have to do with the dependence of head excursions on stride length as discussed
above (Fujita 2004; 2006; Muir and Gowri 2005). From a bio-mechanical point of view
short stride lengths mean that the center of gravity shows fewer deviations from
the position of the ground contacting foot while walking, i.e. there is less need
for balance support from the head.
Although head-bobbing has been studied mainly in pigeons and chicken, several authors
mention this behavior in other species (Dunlap and Mowrer 1930; Daanje 1951; Dagg
1977; Whiteside 1967; Frost 1978: Fujita and Kawakami 2003; Fujita 2006). The most
complete study is by Jimenéz Ortega (2005); it includes 322 species. It seems that
there is some phylogenetic constraint (Dagg 1977; Jimenéz Ortega 2005): usually all
members of the same family or even order show the same behavior (bobbing or not).
Bobbing orders are Columbiformes (pigeons, doves), Galliformes (chickens, pheasants,
quails, peafowl), Gruiformes (cranes, rails) and Ciconiiformes (herons, storks, ibis).
Non-bobbing is found in Sphenisciformes (penguins), Phoenicopteriformes (flamingos),
Pelicaniformes (pelicans, cormorants), Anseriformes (ducks, geese, swans), Falconiformes
(diurnal raptors like hawks, eagles, vultures), Strigiformes (nocturnal raptors like
owls) and Psittaciformes (parrots, cockatoos, budgerigar). Charadriiformes include
shore birds some of which bob (e.g. turnstone, black-winged stilt) and others which
do not (e.g. silver gull, terns) or only occasionally (e.g. black-headed gull, oystercatcher).
Species of the large order of Passeriformes (includes songbirds) generally show hopping
and/or head-bobbing (small birds usually hop; larger birds like magpies both hop
and bob their heads).
Waterfowl with palmate feet seem to be non-bobbing species independent of the order
they belong to (e.g. ducks, flamingos, cormorants, pelicans, gulls). One exception
is the black-headed gull which shows head-bobbing during foraging in the mud but
not when walking on the ground or when swimming. Non-bobbing species generally do
not bob either when walking on land or when swimming. There are water-dwelling species
like coots and common moorhens which bob their heads both when walking on the ground
and when swimming (Videos 3, 4). These species have feet specialized for swimming
but no palmate feet.
Species-dependency of head-bobbing
Not all birds that walk on the ground show head-bobbing. Well-known examples of non-bobbing
birds are waterfowls like ducks, geese and swans (Videos 2, 3)). These species have
relatively short and wide-set legs, which is advantageous while swimming but results
in waddling when walking on the ground. This raises the question why some species
bob their head and others do not. It seems that there is no simple answer to this
question and Dagg (1977), after having listed quite a number of bobbing and non-bobbing
species, notes that it is a puzzle why some birds do it and others do not.
Synchronization of head movements and leg movements
The forward and backward movements of the head are synchronized with the movements
of the legs (Bangert 1960; Frost 1978; Troje and Frost 2000; Fujita 2002; 2003).
When both feet contact the ground the center of gravity is caudal to the rostral
foot. This foot becomes the supporting foot when the caudal foot starts its swing
phase. The thrust is initiated in this phase. This results in a forward shift of
the center of gravity so that it is placed above the supporting foot (Fujita 2002).
In the course of the step the supporting foot moves backward, which means that the
center of gravity moves rostral to this foot. At the same time the head moves backward
(relative to the body) which means that the center of gravity is also shifted backwards
towards the ground foot. In this way the center of gravity seems to follow the supporting
foot which helps to stabilize balance. The same cycle occurs during the stance phase
of the other leg, i.e. there are two cycles of head-bobbing during a complete walking
cycle. This suggests that one function of head-bobbing is to shift the center of
gravity relative to the feet so as to maximize postural stability (Dagg 1977).
Fujita (2002; 2003) studied the contribution of the head to the position of the center
of gravity both in pigeons (Fujita 2002) and in the little egret (Fujita 2003). In
both species this contribution is small but significant. A well-known function of
the head in shifting the center of gravity can be seen in flying birds which stretch
their head to shift the center of gravity towards the wings. Fast running birds usually
stretch their head continuously, which results in a similar forward shift of the
center of gravity which supports the forward movement of the body. On the other hand,
pigeons walking on a treadmill (Frost 1978) and blindfolded pigeons (Necker et al.
2000) which do not bob their heads are able to keep balance when walking. All these
findings show that head-bobbing can help to adjust balance during locomotion but
the contribution seems to be small and keeping balance can be achieved without head-bobbing.
Bangert (1960) studied the development of head-bobbing in chickens and found out
that both head-bobbing and the synchronization of head and leg movements appear within
24 hours after hatching. The cycle frequency decreases with increasing age and size.
Chicks which spent 14 days in a dark room after hatching showed the same synchronization
of head and leg movements as normal chicks of the same age. Restriction of leg movements
for 90 hours after hatching did not impair head-leg synchronization. These experiments
show that the synchronized movements are organized by an endogenous (innate) mechanism.
There is not only a synchronization of head and leg movements but also a dependence
of the extent of the head thrust and stride length. Chicks with restricted locomotor
experience or an experimental decrease of stride length by hobbling for 12 days after
hatching showed both smaller stride lengths and smaller head movements (Muir and
Chu 2002; Muir and Gowri 2005). There even seems to be some correlation between stride
length and head-bobbing under normal conditions. Species with a short stride length
(relative to the height of the hip) like pintails or black-headed gulls show no head-bobbing
as compared to e.g. pigeons and herons with longer stride lengths and distinct head-bobbing
(Fujita 2004). Furthermore, in one and the same species, the black-headed gull, head-bobbing
depends on the stride length, which varies with behavior: while foraging in the mud
the stride length increases and they bob their heads (Fujita 2006).
The hopping or jumping of birds is composed of two phases (Daanje 1951). In the first
phase the legs are folded and the head is retracted. In the second phase both head
and legs are stretched and the bird jumps into the air. Before landing the head is
retracted. Daanje (1951) argues that when walking each step is a hop. In this way
walking would be alternative hopping with both legs, with the head giving mechanical
assistance in the same way as in each hop.
Functional implications for vision
During the hold phase the image of the surrounding world is stabilized for a short
while on the retina, which increases the time to recognize and identify objects,
especially moving ones. This is comparable to fixing an object during self-motion
(slow phase of the optokinetic response).
Most birds have lateral eyes with a minor binocular overlap, which means poor stereoscopic
or depth vision. During monocular vision, motion parallax provides depth information.
Motion parallax is the apparent relative angular movement of objects at different
distances during translatory movements of the eye. It provides unambiguous cues to
the relative depth of stationary objects but not of moving objects. Motion parallax
increases with the velocity of eye movement. In this way the thrust phase may improve
depth information. There is experimental evidence that birds use motion parallax
for depth information (van der Willigen et al. 2002; Cavoto and Cook 2006). Pigeons
head-bob without a hold phase during landing and when running fast which supports
the function of providing depth information (Davies and Green 1988; Green et al.
1994). Cronin et al. (2005) recently confirmed for the whooping crane (Grus americana)
that with increasing walking speed the hold phase decreased and even disappeared.
Davies and Green (1988) suggest that the hold phase serves the recognition of moving
objects and that the thrust phase improves the recognition of stationary food items
like grains. However, in a recent investigation it was shown that shape recognition
of an object was as good in the hold phase as in the thrust phase (Jimenez Ortega
et al. 2009).
Origin of head-bobbing
It has been shown repeatedly that the hold phase is triggered by vision: a hand-held
chicken (Dunlap and Mowrer 1930) or pigeon (Frost 1978) shows head-bobbing when moved
forward by the experimenter. Pigeons walking on a treadmill (stable environment)
do not bob their heads (Frost 1978). Blindfolded animals never show head-bobbing
when walking (Dunlap and Mowrer 1930; Necker et al. 2000 and unpublished own observations).
Friedman (1975) confirmed the visual context of head-bobbing and excluded an influence
of the movement of the legs (no bobbing when legs walk but visual environment is
stable) and of the vestibular system (no bobbing when body moves but visual environment
is stable). Frost (1978) noted that there is a slight forward movement of the head
during the hold phase (about 3 mm/s compared to about 500-800 mm/s during the thrust
phase) which was confirmed later on (Troje and Frost 2000). Such a stimulus is necessary
since an absolutely stable eye position cannot generate an error signal for triggering
head movements as already noted by Dunlap and Mowrer (1930). Altogether head-bobbing
has been compared to the optokinetic response in mammals with a slow motion of the
eye when fixing an object (hold phase) and a rapid saccadic eye movement to fix a
new object (thrust phase). Although head bobbing birds like the pigeon are able to
move their eyes (Nye 1969; Gioanni 1988; Wohlschläger et al. 1993) it seems that
the long neck favors moving the head rather than the eyes.
Conclusions
Although there are now quite a number of observations available, there is still no
unequivocal interpretation of the function of head-bobbing and why some birds bob
their head and others do not. Head-bobbing seems to be an innate mechanism in birds
coupled with the locomotion of the hindlimbs (Bangert 1960). It develops independent
of external stimuli but is under visual control (Friedman 1975; Frost 1978). It seems
that relative stride length plays an important role because head excursions depend
on this parameter and birds which take small steps do not bob. This speaks in favor
of a bio-mechanical component to stabilize balance which is more necessary with long
strides. Birds are bipedal animals with a horizontal orientation of the body and
a center of gravity rostral to the insertion of the hindlimbs which requires an efficient
control of balance when walking on the ground (Necker 2006). Aside from stride length
the role of retinal specialization cannot be excluded. The correlation of a horizontal
retinal band with non-bobbing behavior (Whiteside 1967) fits with many species walking
on the ground.
The hold phase and the thrust phase have been suggested to play different roles as
far as vision is concerned. The hold phase is thought to help in identifying objects,
whereas the thrust phase may improve monocular depth perception. However, these functional
interpretations have not yet been tested experimentally in walking birds. There is
only evidence that birds can see well during all phases of the head-bobbing cycle
(Jimenéz Ortega et a. 2009). Longnecked birds often show thrust and hold phases without
making a step when foraging on the ground (Fujita and Kawakami 2003; own observations
in ostrich, peacock, grey heron, and cranes). In species like coots which bob their
heads while swimming there is no need for a stabilization of balance. These birds
peck for food on the surface of the water, and head-bobbing may improve recognition
of small food items. These examples suggest a visual function of the bobbing behavior.
Altogether it seems that the visual aspect of head-bobbing is the primary function.
Head-bobbing may help in improving object detection when foraging on the ground.
The coordination of head-bobbing and leg movements is not necessary for keeping balance
but may help in stabilizing position during walking. For those birds that practice
head-bobbing, both functions seem to be useful adaptations to cope with walking on
the ground.
Literature update:
Hancock JA, Stevens NJ, Biknevicius R: Elegant-crested Tinamous Eudromia elegans
do not synchronize head and leg movements during head-bobbing. IBIS 157: 198-208
(2014)
Main results: Head and leg movements are coordinated but not synchronized (large
variations); head movements affect stance duration which improves image stabilization;
the center of mass (COM) moves vertically rather than horizontally during head-bobbing.
Video 2: Goose walking without head-bobbing
Video 3: Goose swimming without head-bobbing
Video 4: Common moorhen walking with head-bobbing
Video 5: Common moorhen swimmimg with











